Vascular Regeneration
Rob Allen, Keewon Lee |
 |
Small Diameter Grafts
Objective: This research focuses on rapidly degradable synthetic materials for resorbable vascular grafts. Resorbable vascular grafts must have suitable mechanical properties, good blood compatibility, and resorb at a rate allowing new tissue to replace its function. Our lab’s recent work demonstrates that fast degrading arterial grafts can rapidly remodel into artery-like tissue in small animals.
|
 |
Tissue Engineered Vascular Grafts
Objective: The objective of this project is to engineer arteries (inner diameter ~ 4 mm) by culturing vascular cells on novel elastomeric tubular scaffolds in pulsatile flow bioreactor. We successfully engineered large arterial constructs with substantial mature and circumferentially organized elastin using poly(glycerol sebacate) (PGS) tubular scaffolds cultured primary baboon/porcine smooth muscle cells in newly designed pulsatile flow bioreactor for 3 weeks. Tri-culture with vascular endothelial cells, smooth muscle cells, and fibroblasts in our bioreactor system is the next step to engineer anti-thrombogenic, compliant, and robust arterial constructs.
|
Nerve Regeneration
Eric Jeffries, Britta Rauck |
 |
Peripheral Nerve Repair
Objective:
Develop biodegradable grafts to facilitate peripheral nerve regeneration by mimicking the biochemical and physical environment of natural nerve. We are using electrospinning to create highly porous guides with a fibrous architecture that is similar to extracellular matrix. This is combined with templating to create channels to guide axon growth cone and infiltrating schwann cells.
|
 |
Spinal Cord Repair
Objective:
|
Biomaterial Design
Noah Johnson, Kyubum Kim |
 |
Polycations for Drug Delivery
Objective:
Biocompatible polycations have numerous applications in materials science and medicine. Our lab focuses on the design and synthesis of strong polycation molecules which may interact with heparin, the most negatively charged naturally-occurring polyanion. Complexation with heparin yields a multi-functional drug delivery platform, as heparin binds strongly to many growth factors and cytokines. Upon interaction, the polycation-heparin complex precipitates in aqueous solution, termed a “coacervate” which is similar to an oil emulsion in water. Signaling molecules bound to heparin during precipitation become incorporated into these coacervate droplets which preserve their biological activity. The polycations developed in our lab have also shown the potential to interact with DNA which bears a negative charge, extending their application to the field of gene delivery. The lab has thus far developed two synthetic polycations, polyethylene argininylaspartate digylceride (PEAD) and polyethylene lysinylaspartate dig lyceride (PELD) which have been proven to deliver numerous growth factors in a sustained and controllable fashion.
|
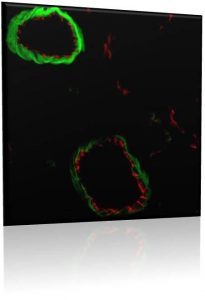
Protein Delivery
William Chen, Kyubum Kim, Noah Johnson, Hassan Awada
|
 |
Cardiac Repair
Objective:
Heart failure is currently one of the leading causes of death in the US and the world. This is due to damaged heart muscle, often a result of a heart attack, and thus the heart can no longer supply an adequate amount of blood to the body. Current therapies seek only to prevent or reduce the damage to the heart, while a true regenerative and reparative solution is needed. We use our polycation-heparin delivery system to release cardiac-implicated growth factors which may protect cardiomyocytes from oxidative stress, incite angiogenesis to alleviate heart ischemia, and induce stem cell homing to the damaged tissue to begin heart repair. The coacervate can be injected directly into the heart wall during open heart surgery, or even by endocardial injection through a cardiac catheter.
|
 |
Wound Healing
Objective:
The US alone sees 100 million acute skin wounds annually, making it one of the most prevalent and economically burdensome healthcare issues. Current standards of care include frequent dressing changes and a heightened risk of infection until the barrier function of the skin is regained. Growth factors orchestrate the wound healing process, thus their strategic application is a promising wound healing therapy. Our lab has shown that controlled delivery of heparin-binding epidermal growth factor (HB-EGF) using the polycation-heparin coacervate can significantly accelerate the healing process, thus reducing inpatient time and cost and risks of infection. HB-EGF speeds up re-epithelialization, granulation tissue formation, and can reduce scar formation following healing. The coacervate can be applied directly to the wound as a liquid, in a cream, or can be coated onto standard bandages. The lab is currently investigating the potential to treat chronic “non-healing” wounds, such as diabetic skin ulcers and pressure ulcers.
|
Bone Regeneration
Noah Johnson |
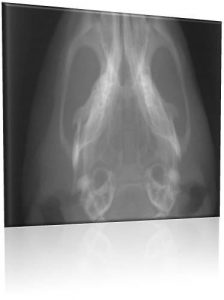 |
Bone Regeneration
Objective:
Bone defects resulting from trauma, tumor, and other diseases are an important health care problem that present a significant clinical challenge. Our lab takes a true tissue engineering approach, using scaffolds, cells, and growth factors for bone regeneration. Our functionalizable polymeric scaffolds improve the osteogenetic function of osteoblasts and stem cells. We also collaborate with Pitt,s Stem Cell Research Center (SCRC), using our polycation-heparin system to sustain the release of bone morphogenetic protein-2 (BMP-2) which stimulates their muscle-derived stem cells (MDSCs) to form bone. This approach has led to complete healing of bone defects in mice and may therefore represent a promising bone regeneration therapy.
|









